Redox cycling of iron/iron oxide has many potential applications for gas-phase reactions and electrochemical devices, including intermediate temperature (550-800°C) iron-air batteries [1,2]. The major limitation to such technologies is the pulverization and sintering of the iron substrate with repeated phase transformation-induced volume changes and consequent mechanical stresses.
By alloying the redox-active Fe, a variety of new behaviors emerge that can be used to extend the lifetime of the material overall. By observing the microstructure of different alloys in the reduced state, the oxidized state, or in between we can better understand how the alloying element influences the microstructure and how microstructural evolution impacts the architecture of the sample as a whole. For instance, we have found that in the presence of a redox-inactive, Fe-soluble metal such as Ni or Co, the reduction reaction is accelerated and pores formed during cycling sinter close much more readily than in the pure Fe foam [3,4,5]. Current work focuses on the influence of different architectures, such as ink-printing or bridging fibers, on the longevity of Fe foams.
Our characterization techniques include metallography, SEM, operando X-ray diffraction, and X-ray computed tomography.
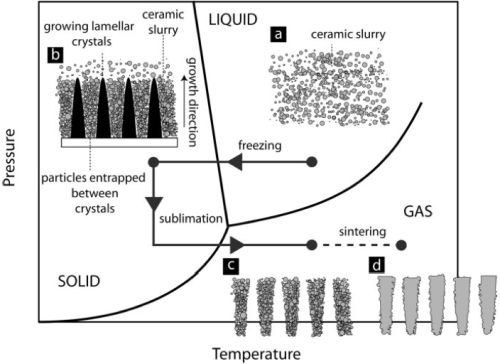
Fig. 1. Phase diagram of water, showing the process steps of freeze casting [4].
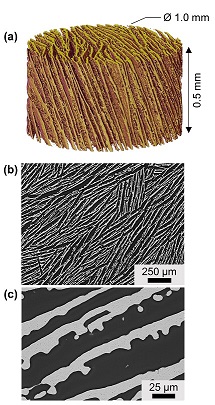
Fig. 2. Freeze-cast iron foams comprising dense, iron lamellae separated by macropores. (a) A cylindrical volume imaged using X-ray microtomography, (b) optical micrograph of cross-section perpendicular to freezing direction, and (c) electron micrograph at higher magnification.

Fig. 3.Electron image of a Fe-10W foam in the oxidized state, showing porous interior with thick Fe3O4 scale along free surfaces.
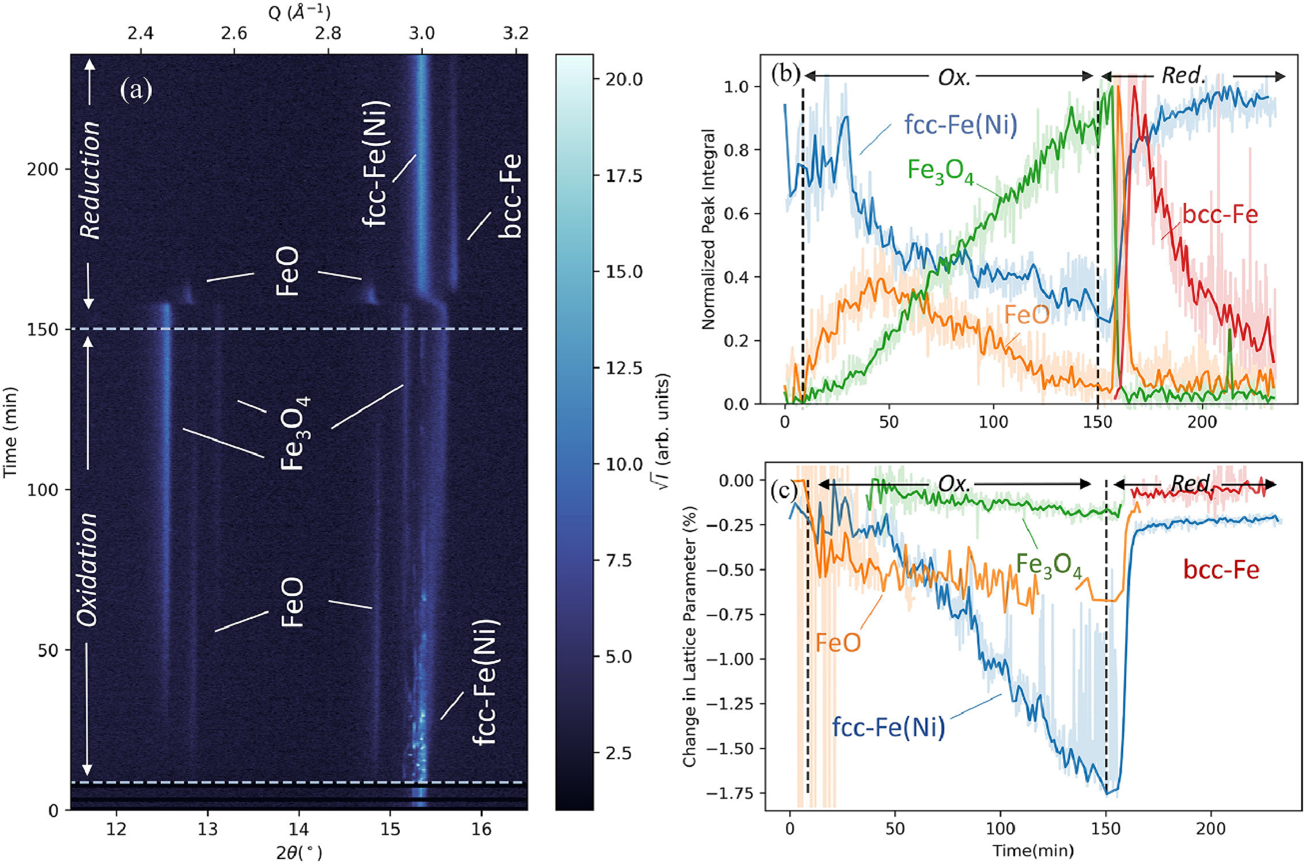
Fig. 4.Operando XRD results for an Fe-25Ni foam, showing accelerated reduction kinetics. Reproduced from [3].
Related Publications
- C.M. Berger, O. Tokariev, P. Orzessek, A. Hospach, Q. Fang, M. Bram, W.J. Quadakkers, N.H. Menzler, H.P. Buchkremer, Development of storage materials for high-temperature rechargeable oxide batteries, J. Energy Storage. 1 (2015) 54-64. doi:10.1016/j.est.2014.12.001.
- Q. Fang, C.M. Berger, N.H. Menzler, M. Bram, L. Blum, Electrochemical characterization of Fe-air rechargeable oxide battery in planar solid oxide cell stacks, J. Power Sources. 336 (2016) 91-98. doi:10.1016/j.jpowsour.2016.10.059.
- Mack, Jacob B., Samuel M. Pennell, and David C. Dunand. "Microstructural evolution of lamellar Fe-25Ni foams during steam-hydrogen redox cycling." Acta Materialia 237 (2022): 118148.
- Pennell, Samuel M., Jacob B. Mack, and David C. Dunand. "Evolution of lamellar architecture and microstructure during redox cycling of Fe-Co and Fe-Cu foams." Journal of Alloys and Compounds 918 (2022): 165606.
- Mack, Jacob, Samuel M. Pennell, and David C. Dunand. "Sintering Inhibition Enables Hierarchical Porosity with Extreme Resistance to Degradation During Redox Cycling of Fe-Mo Foams." Available at SSRN 4347875.
- Pennell, Samuel, and David Dunand. "Effects of bridging fibers on the evolution of lamellar architecture during H2/H2O redox cycling of Fe-foams." Acta Materialia 243 (2023): 118543.
